ANGEW | Researchers Developed Photo-assisted One-pot Strategy for Convergent Synthesis of a Highly Branched Heptadecasaccharide with Anti-Pancreatic Cancer Activity
Update time:2022-07-01
Carbohydrates are ubiquitous biopolymers in nature and play key roles in many biological processes. The microheterogeneity and structural complexity of carbohydrates make it difficult or even impossible to obtain from natural sources in acceptable purity and sufficient quantity, which severely restricts their study at molecular level and hinders their potential applications in biomedical field. Alternatively, chemical synthesis provides access to homogeneous and well-defined oligosaccharides or polysaccharides. However, efficient synthesis of polysaccharides and verification of their active domains remain a major challenge for the development of polysaccharides drugs.
In their previous work, the research group of DING Kan from the Zhongshan Institute for Drug Discovery, found that the polysaccharide HH1-1 isolated from Carthamus tinctorius L. could inhibit the growth of pancreatic cancer cells in vivo and in vitro. However, the effective concentration of HH1-1 required in vivo is about 50 times lower than that required in vitro. Therefore, the research team speculated that HH1-1 might be degraded and metabolized into several fragments in vivo, and then exert its anti-pancreatic cancer activity. However, the precise active domains of HH1-1 that truly exert their biological activities have yet to be verified.
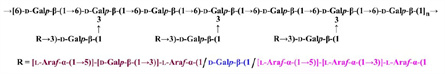
Figure 1. The putative structure of polysaccharide HH1-1.
In a recent study published in Angewandte Chemie International Edition (IF=16.823), the team of DING Kan in collaboration with the research group led by LI Tiehai from SIMM and Prof. WEI Bangguo from School of Pharmacy of Fudan University, reported the first total synthesis of a highly branched heptadecasaccharide moiety of the native bioactive polysaccharide HH1-1, as well as their short fragments, via a photo-assisted one-pot glycosylation strategy.
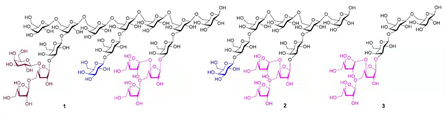
Figure 2. Synthetic heptadecasaccharide 1 and its short fragments 2-3.
In this strategy, the photoremovable o-nitrobenzyl protecting group to in situ generate acceptor after ultraviolet radiation enables the streamline assembly of heptadecasaccharide by the use of sequential glycosylations in a convergent [6+4+7] one-pot manner, during which the aglycon transfer was eliminated because the sequence of assembly is from reducing end to non-reducing end of the polysaccharide.
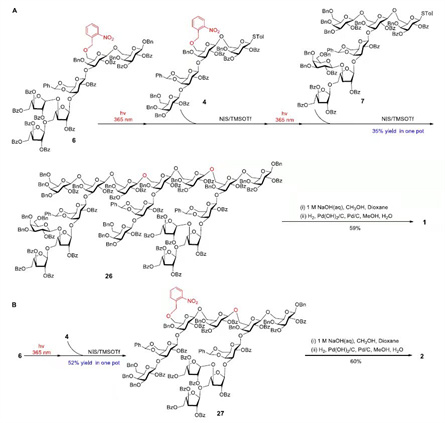
Figure 3. Synthesis of heptadecasaccharide 1 and decasaccharide 2.
The bioactivity tests showed that compounds 3 indeed might bind to galectin-3 well (KD value = 6.93E-4), and the inhibitory ratio of compound 3 on AsPC-1 cell line can be up to 62% at 20 μM dosage. The data suggested that compound 3 might be the active domain of the native polysaccharide HH1-1 against pancreatic cancer cells, which could be a novel leading compound for new drug candidate development against pancreatic cancer. The deep bioactivity evaluation and the mechanism study are ongoing.
This work demonstrates a representative example to understand the active domains of the polysaccharide, which could be synthesized for structure-activity relationship studies, allowing for further structure modification and potential drug candidate development.
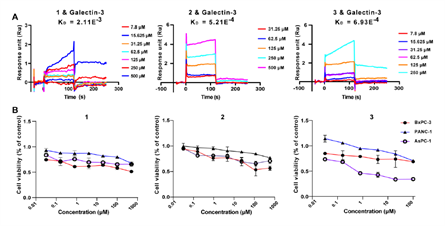
Figure 4. Compounds 1-3 target galectin-3 to inhibit pancreatic cancer cell growth.
DOI: https://doi.org/10.1002/anie.202202554
Link to article: https://onlinelibrary.wiley.com/doi/10.1002/anie.202202554
Contributing Department: Research Group of DING Kan
