NEUROBIOL DIS | Scientists Constructed A Novel Mouse Model with Kcnq2-R207W Mutation Displaying Epileptic Phenotype and Response to the Novel Anti-epileptic Drug
Update time:2022-09-14
Epilepsy is a neurological disorder caused by the sudden abnormal discharge of brain neurons. Epilepsy is a chronic and disabling disease that seriously threatens human life and health. KCNQ2 voltage-gated potassium channel is a confirmed pathogenic gene for epilepsy. Its gene mutation can lead to severe developmental and epileptic encephalopathy.
In a recent study published in Neurobiology of Disease (IF=7.046), the team of GAO Zhaobing from the Zhongshan Institute of Drug Discovery , in collaboration with Maurizio Taglialatela from the Federico II University of Napoli (Italy), reported a novel mouse model harboring the Kcnq2 R207W voltage-sensor mutation, which displayed spontaneous seizure and sensitivity to HN37, the new generation of anti-epileptic drug targeting the KCNQ2.
|
|
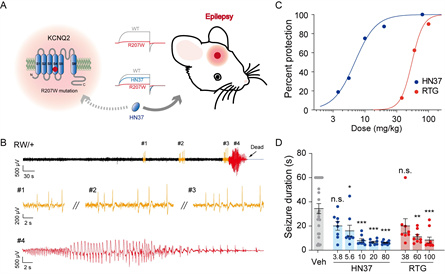
Establishment of a novel mouse model harboring Kcnq2 R207W voltage-sensor mutation, which displays spontaneous seizure and sensitivity to HN37, the new generation of anti-epileptic drug targeting the KCNQ2 channel
KCNQ2 voltage potassium channel is highly expressed in the central nervous system and plays an important role in the regulation of nerve excitability; its gene mutation can cause rare genetic epilepsy. Currently, hundreds of pathogenic mutations of KCNQ2 have been reported clinically, and most of them are missense mutations in severe patients, mainly distributed in key structural domains such as the fourth transmembrane region (S4) responsible for voltage sensing and pore formation region. Gene-edited mice are an important tool for the study of genetic diseases. In previous studies on KCNQ2 mutation-related epilepsy, scientists mostly chose the pore region mutation to construct gene-edited mice without involving the voltage-sensitive region. At the same time, most KCNQ2 point mutant mice did not have spontaneous seizures and could not fully simulate the disease phenotype of epileptic patients.
In the previous work, the team conducted functional screening of a large number of pathogenic mutations of KCNQ2 and found that R207W, the S4 transmembrane mutation that frequently appeared in the patient cohort, had unique biophysical characteristics, namely, voltage-dependent rightward-shift and slow opening dynamics, so it was classified as loss-of-function mutation.
In this study, the team successfully obtained KCNQ2-R207W point mutation mice by CRISPR/Cas9 technology. After systematic EEG analysis and behavioral monitoring, it was found that KCNQ2-R207W mice had spontaneous epileptic seizures, manifested as convulsions, myoclonus, generalized tonic, etc. This is the first global report of spontaneous seizures in mice caused by mutations in the KCNQ2 voltage sensing region. At the same time, the team evaluated the epilepsy susceptibility of the mutant mice by applying various inducible factors and found that the mice showed decreased epilepsy threshold in response to noise, high fever, and electrical and chemical stimuli, suggesting that the mutation of KCNQ2 has a significant impact on the neural excitability of the mouse brain. Finally, the team treated epileptic mice with the new antiepileptic drug pynegabine, and the results showed that the epileptic phenotype of mice was alleviated after treatment, suggesting that KCNQ2 channel agonists may have targeted therapeutic effects on this type of epilepsy. The anti-epileptic drug pynegabine was independently developed by the Shanghai Institute of Materia Materia Sinica, Chinese Academy of Sciences, and its domestic rights and interests have been transferred to the pharmaceutical company. At present, the phase Ia clinical trial (CTR20201676) for this drug has been completed, demonstrating good safety and meabolic characteristics.
DOI:https://doi.org/10.1016/j.nbd.2022.105860
Link to article:
https://www.sciencedirect.com/science/article/pii/S0969996122002522?via%3Dihub
Contributing Department: Research Group of GAO Zhaobing